Market Accelerator combines the full power of our Unified Services Platform to streamline every stage of commercialization. From scaling production to navigating regulatory approvals, we ensure your path to market is seamless, efficient, and fully optimized. With Benuvia, quick-to-market doesn’t mean cutting corners—it means doing it right the first time.
With a flexible and collaborative approach, we offer a variety of partnership models designed to meet the unique needs of our clients and maximize the potential of every opportunity. Whether through licensing agreements, co-development projects, supply partnerships, or global distribution strategies, we work hand-in-hand with our partners to deliver exceptional results.
Strategic Partnerships foster collaboration through co-creation and shared decision-making. With profit-sharing opportunities, this pathway aligns our goals with yours, creating a dynamic framework to innovate together, share risks, and maximize the success of every project.
Schedule a Meeting
Benuvia’s end-to-end managed services provide seamless solutions for clients without the expertise to execute critical stages of development. From formulation and testing to production and scaling, we ensure precision, compliance, and efficiency, guiding your product to success.
Schedule a Meeting
Pre-validated monographed profiles simplify drug development by reducing complexities, accelerating timelines, and streamlining regulatory submissions. With ready-to-use formulations and clear pathways for scaling, this program offers a strategic advantage for rapid market entry.
Schedule a Meeting
With pre-validated monographs and expertise in controlled substances, sublingual formulations, and advanced manufacturing, we accelerate your path to market. From regulatory guidance to scalable production, our program delivers precision, compliance, and efficiency at every stage of development.
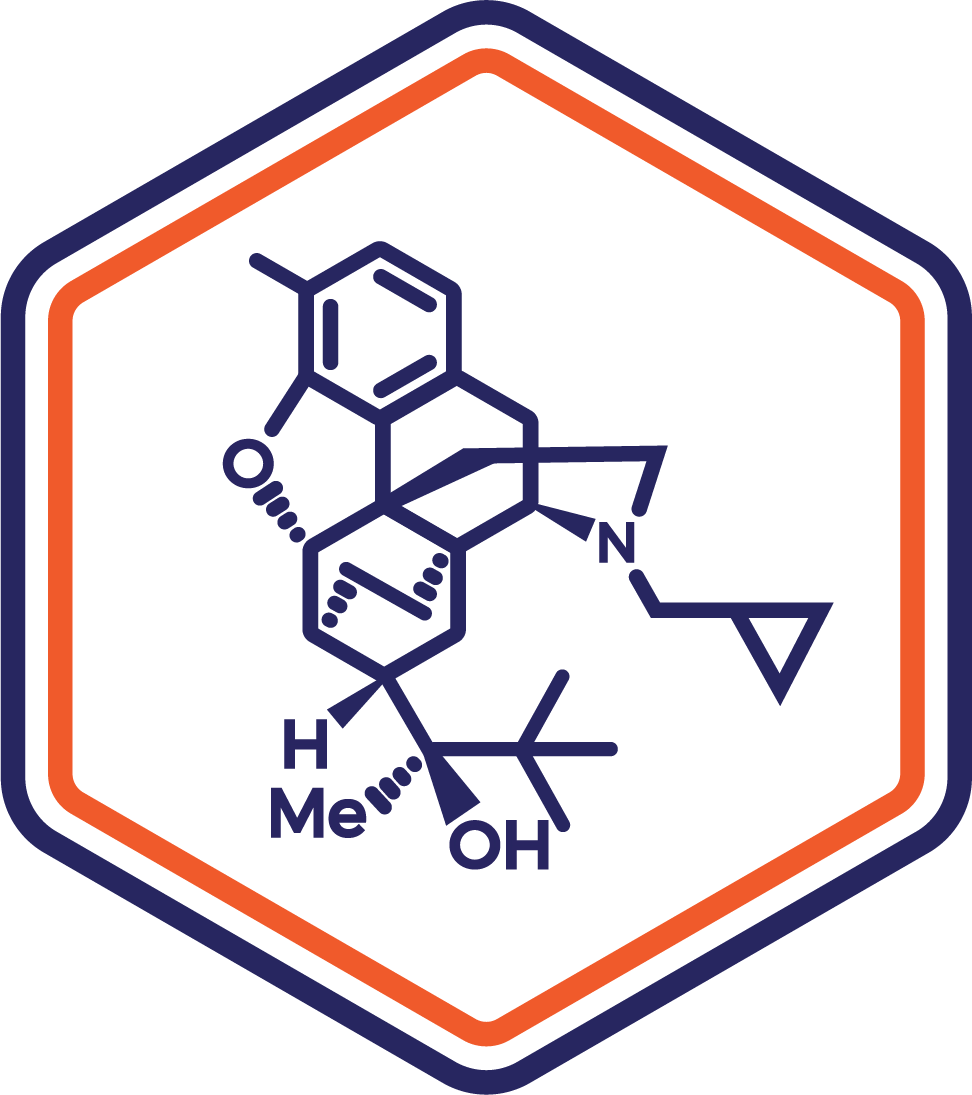
Indication: Moderate to Severe Pain
Clinical Stage: Registration
A sublingual formulation of buprenorphine designed for precise dosing and rapid absorption, offering an optimized delivery method for treating moderate to severe pain. This formulation targets unmet needs in pain management by minimizing first-pass metabolism and enhancing bioavailability.
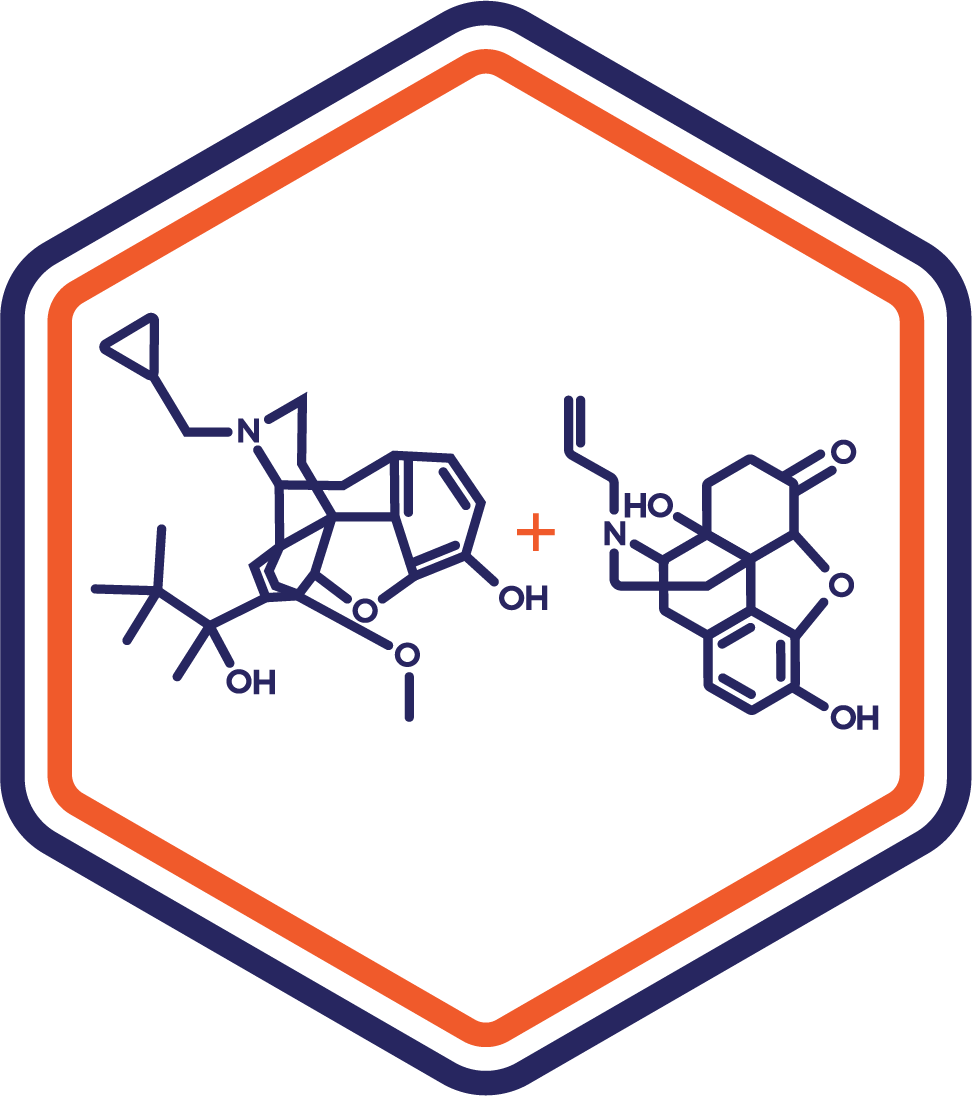
Indication: Opioid Withdrawal
Clinical Stage: Phase 2
This combination therapy leverages buprenorphine’s analgesic and withdrawal management properties alongside naloxone’s abuse-deterrent mechanism, enhancing compliance and safety, making it a compelling option for opioid dependency.
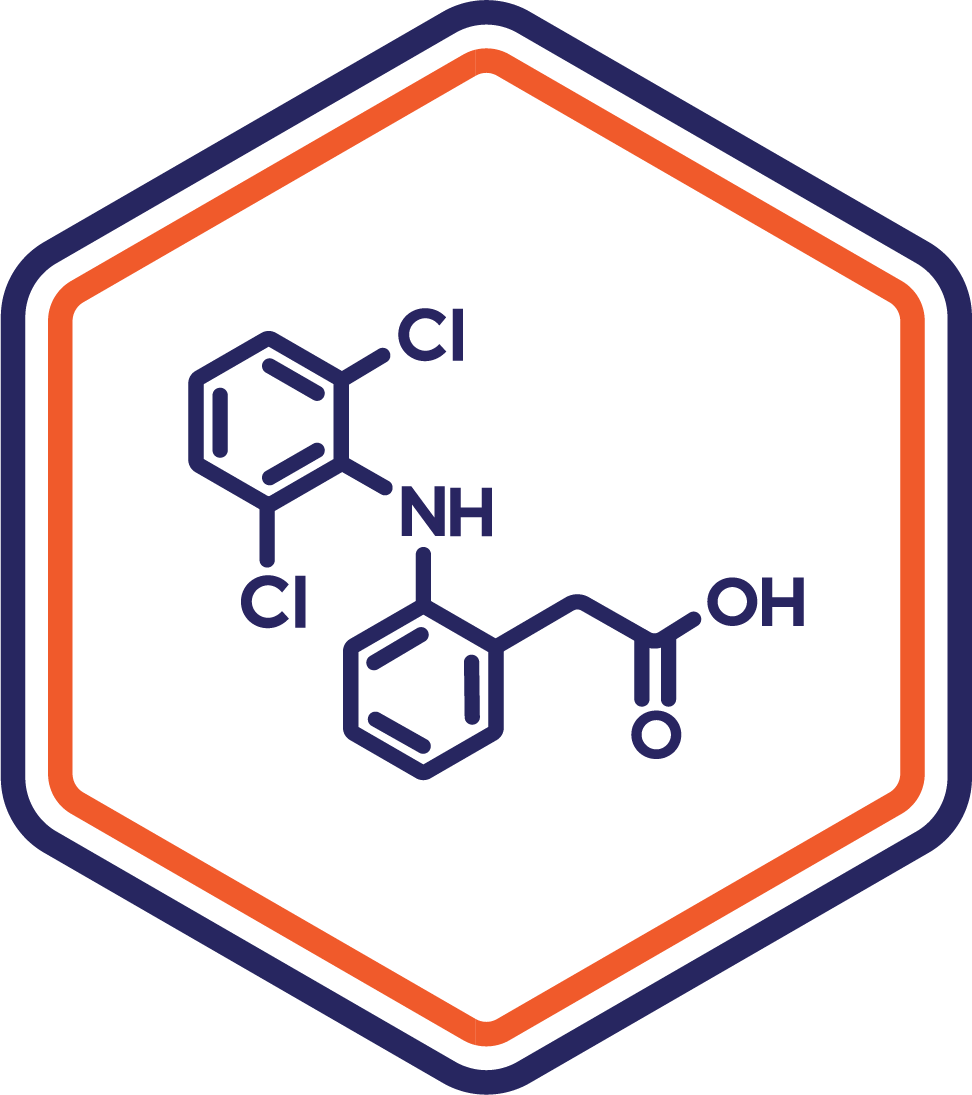
Indication: Pain
Clinical Stage: Phase 2
A reformulated version of diclofenac designed for sublingual delivery, offering a rapid-onset alternative to traditional oral formulations. This innovative solution minimizes gastrointestinal irritation while enhancing patient convenience and compliance.
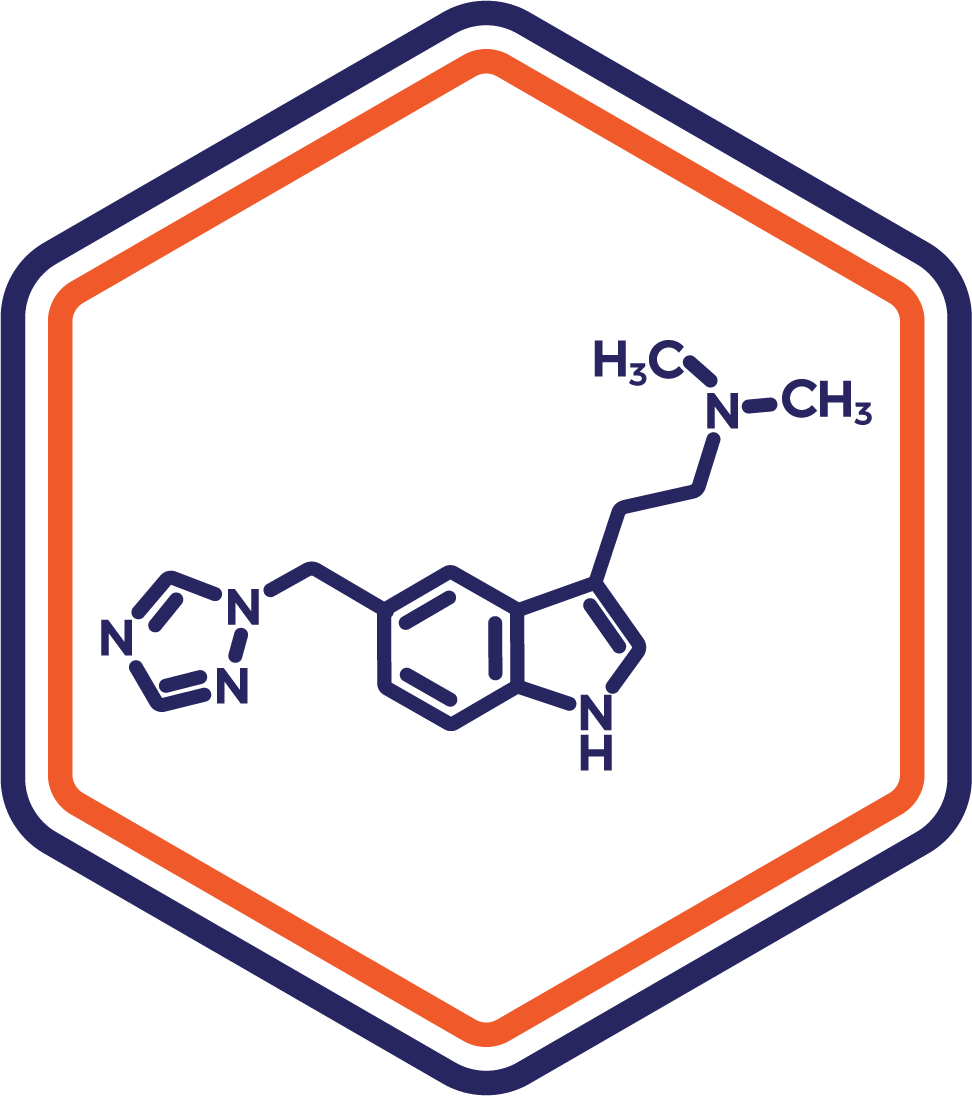
Indication: Migraine
Clinical Stage: IND
Designed to deliver rapid relief for acute migraines, this sublingual rizatriptan formulation bypasses gastrointestinal delays for faster symptom resolution. It’s a strategic alternative to current treatments, targeting patients with nausea or difficulty swallowing tablets.
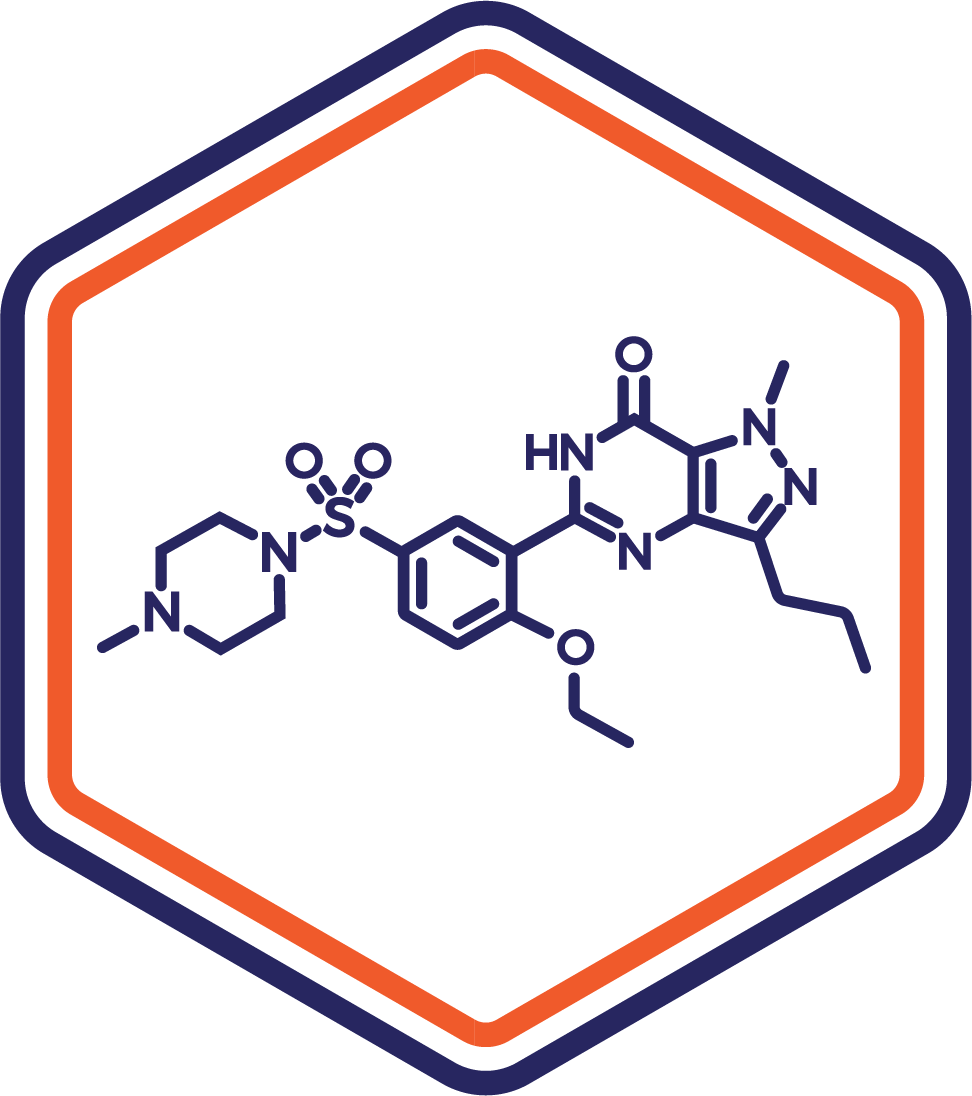
Indication: Erectile Dysfunction/PAH
Clinical Stage: Phase 2
A novel sublingual formulation of sildenafil offering enhanced onset of action compared to oral tablets. This innovation supports improved patient adherence and expands therapeutic applications, including pulmonary arterial hypertension (PAH).
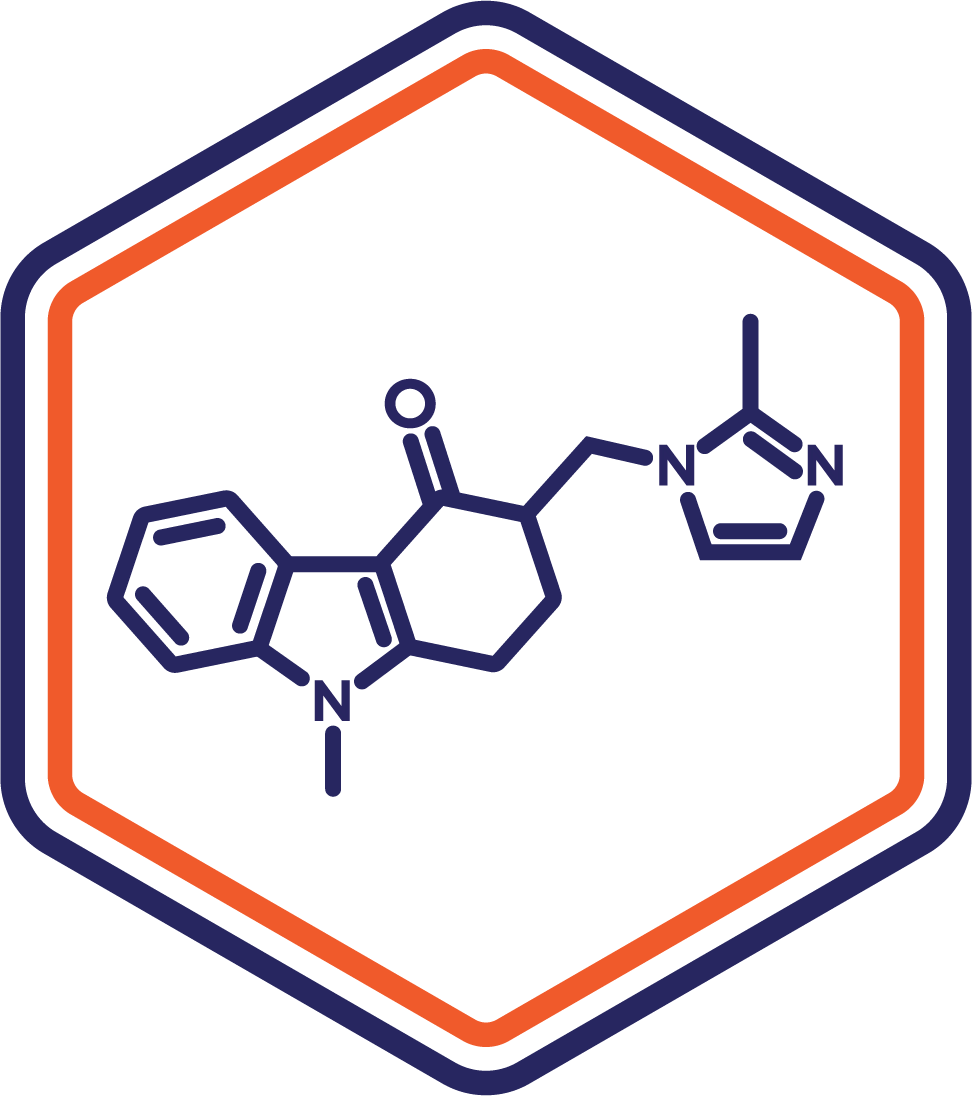
Indication: Nausea/Vomiting (Chemotherapy)
Clinical Stage: Phase 1
This sublingual formulation of ondansetron provides faster antiemetic relief for chemotherapy-induced nausea and vomiting. Its ease of use and rapid action address critical gaps in patient care during oncology treatments.
Market Accelerator got our product to market quickly and efficiently. From scalable manufacturing to precise serialization and packaging, their team ensured every step met the highest standards of quality and compliance. Their seamless integration of services and proactive support allowed us to focus on our launch strategy. We couldn’t have achieved this timeline without them.
Global Pharmaceutical Company
Vice President of Commercial Operations

Joe brings over a decade of experience in GMP manufacturing, supply chain optimization, and project leadership. He specializes in the commercialization of APIs and finished drug products, plant-based therapies, and controlled substances, while driving cost efficiencies and ensuring compliance with FDA and DEA regulations.
"*" indicates required fields